Answer:
29.80 grams is the minimum quantity of 20° C water needed to dissolve all the KCl from the sample.
Step-by-step explanation:
Mass of impure sample of
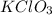 = 40 g
= 40 g
Percentage of KCl impurity sample = 19%
Mass of KCl impurity sample = 19% of 40 g =
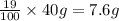
Solubility of KCl in 100 grams of water at 20° C = 25.5 g.
Mass of water required dissolve of 1 gram KCl =
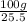
The mass of water required dissolve 7.6 g of KCl at 20°C=
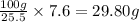
29.80 grams is the minimum quantity of 20° C water needed to dissolve all the KCl from the sample.