Answer: The initial concentration of
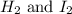 are 0.0192 M and 0.0192 M respectively.
are 0.0192 M and 0.0192 M respectively.
Step-by-step explanation:
We are given:
Equilibrium concentration of HI = 0.030 M
Moles of hydrogen gas = Moles of iodine gas (concentration will also be the same)
For the given chemical equation:
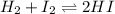
Initial: x x -
At eqllm: x-c x-c 2c
Calculating the value of 'c'
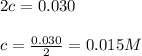
The expression of
 for above reaction follows:
for above reaction follows:
![K_(eq)=([HI]^2)/([H_2]* [I_2])](https://img.qammunity.org/2020/formulas/chemistry/college/9dx6n4gy6wm9q9a8adxuv8ly2l4uf7dtda.png)
We are given:
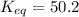
![[H_2]=(x-c)=(x-0.015)](https://img.qammunity.org/2020/formulas/chemistry/college/12ep0hhlrojfi7phvokgocn6mcr032tq3e.png)
![[I_2]=(x-c)=(x-0.015)](https://img.qammunity.org/2020/formulas/chemistry/college/x214tum2efbklaf8ooce6kucs1hkr9682f.png)
Putting values in above equation, we get:
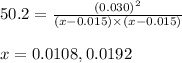
Neglecting the value of x = 0.0108 M, because the initial concentration cannot be less than the equilibrium concentration.
x = 0.0192 M
Hence, the initial concentration of
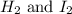 are 0.0192 M and 0.0192 M respectively.
are 0.0192 M and 0.0192 M respectively.