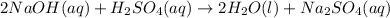Answer:
Step-by-step explanation:
Let's correct and balance the given equation:
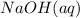 is sodium hydroxide, charge of sodium is +1, charge of hydroxide is -1, so it's fine;
is sodium hydroxide, charge of sodium is +1, charge of hydroxide is -1, so it's fine;
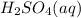 is sulfuric acid, charge of hydrogen cation is +1, charge of sulfate is -2, sulfate is balanced by the two protons;
is sulfuric acid, charge of hydrogen cation is +1, charge of sulfate is -2, sulfate is balanced by the two protons;
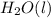 is firstly a molecule, it's water, in its liquid state, the oxidation state of +1 for the two hydrogens is balanced by the oxidation state of -2 for oxygen;
is firstly a molecule, it's water, in its liquid state, the oxidation state of +1 for the two hydrogens is balanced by the oxidation state of -2 for oxygen;- sodium sulfate should be
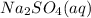 , since sulfate has a charge of -2, this would require two sodium cations to balance it.
, since sulfate has a charge of -2, this would require two sodium cations to balance it.
The equation becomes:
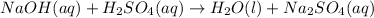
We require 2 NaOH in order to balance the two sodium cations on the right, this would yield a total of 4 hyrogens on the left, so we also need two water molecules to balance it fully: