Answer:
(a) 0.699 kJ/K
(b) -0.671 kJ/K
(c) 0.028 kJ/K
Step-by-step explanation:
The Refrigerant-134a flows into the evaporator as a saturated liquid-vapor mixture and flows out as a saturated vapor at a saturation pressure of 160 kPa and temperature of -15.64°C (estimated from the Saturated Refrigerant-134a Temperature Table).
(a) The entropy change of the refrigerant (ΔS
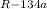 ) = Q/T
) = Q/T

Q = 180 kJ
T
 = -15.64 + 273.15 = 257.51 K
= -15.64 + 273.15 = 257.51 K
ΔS
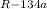 = Q/T
= Q/T
 = 180/257.51 = 0.699 kJ/K
= 180/257.51 = 0.699 kJ/K
(b) The entropy change (ΔS
 ) of the cooled space (ΔS
) of the cooled space (ΔS
 ) = -Q/T
) = -Q/T

Q = -180 kJ
T
 = -5 + 273.15 = 268.15 K
= -5 + 273.15 = 268.15 K
ΔS
 = Q/T
= Q/T
 = -180/268.15 = -0.671 kJ/K
= -180/268.15 = -0.671 kJ/K
(c) The total entropy change for this process (ΔS
 ) = ΔS
) = ΔS
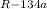 + ΔS
+ ΔS
 = 0.699 - 0.671 = 0.028 kJ/K
= 0.699 - 0.671 = 0.028 kJ/K