Answer:
a. closer to 20∘C
Step-by-step explanation:
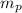 = mass of pallet = 50 g = 0.050 kg
= mass of pallet = 50 g = 0.050 kg
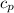 = specific heat of pallet = specific heat of iron
= specific heat of pallet = specific heat of iron
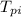 = Initial temperature of pellet = 200 C
= Initial temperature of pellet = 200 C
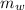 = mass of water = 50 g = 0.050 kg
= mass of water = 50 g = 0.050 kg
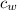 = specific heat of water
= specific heat of water
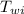 = Initial temperature of water = 20 C
= Initial temperature of water = 20 C
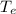 = Final equilibrium temperature
= Final equilibrium temperature
Also given that
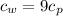
Using conservation of energy
Energy gained by water = Energy lost by pellet
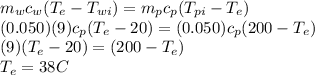
hence the correct choice is
a. closer to 20∘C