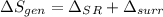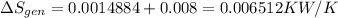Answer:
a)
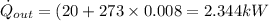
b) T_2 = 36.4 degree C
c)
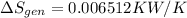
Step-by-step explanation:
Given data:
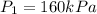
volumetric flow
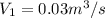
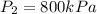
power input
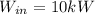
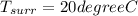
entropy = 0.008 kW/K
from refrigerant table for P_1 = 160 kPa and x_1 = 1.0
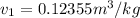
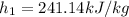
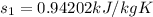
a) mass flow rate
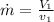
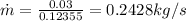
heat loss
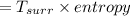
heat loss
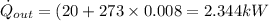
b) from energy balance equation
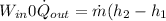
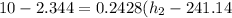
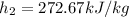
from refrigerant table, for P_2 = 800 kPa and h_2 = 272.67 kJ/kg
T_2 = 36.4 degree C
c) from refrigerant table P_2 = 800 kPa and h_2 = 272.67 kJ /kg
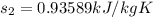
rate of entropy
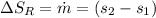
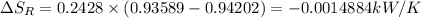
rate of entropy for entire process