Answer:
Molarity of a 5.0 L solution that contains 0.5 moles of KNO3 is 0.1 M
Step-by-step explanation:
Molarity : It is used to express the concentration of the solution and defined as total moles of solute present in one liter of solution .
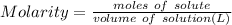
Moles of KNO3 = 0.5 (given)
Volume of solution = 5.0 L
Substitute the value in given formula ,
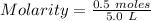
on calculation,
Molarity = 0.1 mol/L
Molarity = 0.1 M
(M = mol/L)