Answer:
2.00976
Step-by-step explanation:
B = Magnetic field = 1.99 T
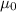 = Vacuum permeability =
= Vacuum permeability =
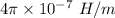
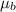 = Magnetic moment of each electron =
= Magnetic moment of each electron =
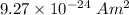
n = Number density of atoms =
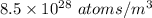
N = Number of electrons per atom
Magnetic field is given by
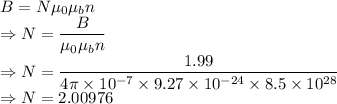
The number of electrons per atom contribute to the saturated field of iron is 2.00976