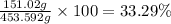Answer:
The percentage by mass of sodium in the alloy is 33.29%.
Step-by-step explanation:
Volume of hydrogen gas =
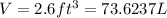
Pressure of hydrogen gas = P = 1 atm
Temperature of the gas = T = 0.0°C =273.15 K
Moles of hydrogen gas = n
 (Ideal gas)
(Ideal gas)
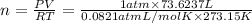
n = 3.2830 mole
Moles of hydrogen gas = 3.280 mole
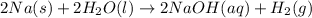
According to reaction 1 mole of hydrogen is obtained from 2 moles of sodium.
Then 3.280 moles of hydrogen gas will be obtained from :
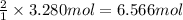
Mass of 6.566 moles of sodium =
6.566 mol × 23 g/mol = 151.02 g
Mass of hydrone = 1.0 lb = 453.592 g
The percentage by mass of sodium in the alloy: