Answer:
5.3072 Liters of volume of ethanol is required.
Step-by-step explanation:
Volume of isooctane = 1 gal = 3.785 L
3.785 L =3.785 × 1000 mL= 3,785 mL
Energy density of isooctane = 32.9 kJ/mL
Energy produced on combustion of 3,785 mL of isooctane :
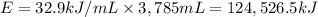
Moles of ethanol which will produce same amount of energy 'E' as 1 gallon of iosooctane = n
Ethanol’s enthalpy of combustion =
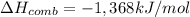
Energy released when 1 mole of ethanol is combusted = 1,368 kJ/mol
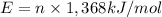
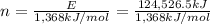
n = 91.03 mole
Mass of 91.03 moles of ethanol , m= 91.03 mol × 46 g/mol = 4,187.38 g
Volume of ethanol = V
Density of ethanol = d = 0.789 g/mL
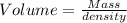
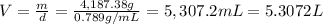
5.3072 Liters of volume of ethanol is required.