Answer:
The following answer are based only on emf values
a).Most anodic element ,Titanium - Ti
b).Metal That will corrode easily is : Titanium - Ti
c). Most reactive metal is Titanium-Ti
d).Al can't be used for making implants because it undergo corrosion easily and do not form strong passive oxide layer.
Ti forms very strong passive layer which prevents further corrosion.
Au is least reactive and does not corrode.
Note : You have to give answer according to E values
- In actual(without considering E values) most anodic, reactive , corrosive element is Al
Explanation:
Here the oxidation potential data is given (since elements are getting oxidised)
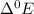 : Standard Reduction Potential : It is tendency of the element to gain electron and get reduced.
: Standard Reduction Potential : It is tendency of the element to gain electron and get reduced.
Oxidation Potential is opposite to reduction potential
Standard Oxidation Potential :It is tendency of the element to loose electron and get oxidised.
It is measured in Volts/V
- More positive (greater)the value of the oxidation potential , stronger is the tendency of the element to get oxidised.
- More negative(lesser) the value of oxidation potential lesser the tendency of the element to get oxidised. Hence the element undergo Reduction in comparison to other with more positive value .
The main application
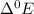 values is in electrochemical cell to predict the products of the reaction. or predict whether the reaction is spontaneous or not.
values is in electrochemical cell to predict the products of the reaction. or predict whether the reaction is spontaneous or not.
- Anodic means tendency to undergo oxidation
Since
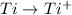 has maximum value of
has maximum value of
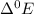 = 2.00V , so it get oxidised as compared to other elements.
= 2.00V , so it get oxidised as compared to other elements.
- Corrode , The metal which oxidise easily will also undergo corrosion easily
From the , E values Ti show maximum tendency of oxidation hence it also get corroded easily.
- Ti is the most reactive because it oxidised fast as compare to other elements
- Au (gold) is the least reactive element as its
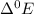 value is negative. So gold don't get corrode and used for making implants. Ti can also be used , since it is most reactive so it react with oxygen present in atmosphere and form a passive layer of oxide which prevent its corrosion. Al also form passive oxide but form very thin layer which is not able to protect it from corrosion
value is negative. So gold don't get corrode and used for making implants. Ti can also be used , since it is most reactive so it react with oxygen present in atmosphere and form a passive layer of oxide which prevent its corrosion. Al also form passive oxide but form very thin layer which is not able to protect it from corrosion