Answer:
1. The correct answer is option a.
2. The correct answer is option B.
3. The correct answer is option C.
Step-by-step explanation:
1. When acid reacts with base heat is generated along with formation of salt and water.
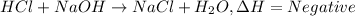
Those reaction in which heat released as a product is called exothermic reaction.Exothermic reaction have negative value of enthalpy of reaction
2.
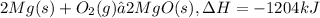
If we reveres the equation we will have the reaction in which MgO is getting decomposed into Mg and oxygen gas.
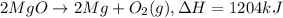
Divide the whole equation by 2.
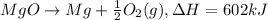
602 kJ is the enthalpy for the decomposition of 1 mole of MgO(s).
3.
The enthalpy for the formation of 1 mole of liquid ammonia = -80.29 kJ/mol
So, enthalpy of formation of 3 moles of liquid ammonia :
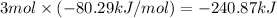
-240.87 kJ is the enthapy for the formation of 3 moles of liquid ammonia.