Answer:
The heat energy transfer required to raise the temperature of 13.0 kg liquid ammonia from -50.0 °C to 0.0 °C is 36,896.44 kJ.
Step-by-step explanation:
The process involved in this problem are :
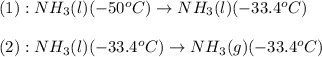
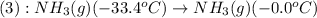
Now we have to calculate the amount of heat released or absorbed in both processes.
For process 1 :
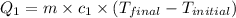
where,
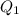 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of ammonia = 13000 g
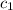 = specific heat of liquid ammonia =
= specific heat of liquid ammonia =
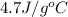
 = initial temperature =
= initial temperature =
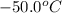
 = final temperature =
= final temperature =
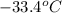
Now put all the given values in
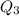 , we get:
, we get:
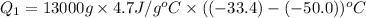
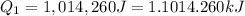
For process 2 :
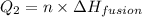
where,
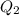 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of solid ammonia = 13.0 Kg = 13000 g
n = Moles of ammonia =
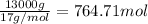
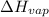 = enthalpy change for vaporization=23.5 kJ/mol
= enthalpy change for vaporization=23.5 kJ/mol
Now put all the given values in
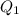 , we get:
, we get:
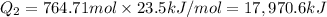
For process 3 :
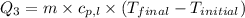
where,
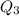 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of ammonia = 13000 g
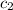 = specific heat of gaseous ammonia =
= specific heat of gaseous ammonia =
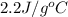
 = initial temperature =
= initial temperature =
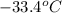
 = final temperature =
= final temperature =
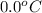
Now put all the given values in
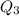 , we get:
, we get:
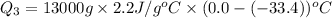
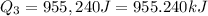
The heat energy transfer required to raise the temperature of 13.0 kg liquid ammonia from -50.0 °C to 0.0 °C = Q
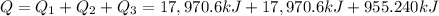
Q = 36,896.44 kJ