Answer:
Correct answer - wheels
Step-by-step explanation:
Limiting reactant:
The reactant that is used up first in the chemical reaction.i.e. the reactant that restricts the amount of product that can be produced.The unused product left in the reaction.
For example,
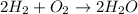
The ratio of creating
 is
is
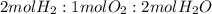
If you had 8 moles of hydrogen and only 3 moles of oxygen ;you would have enough oxygen to produce 6 moles of water with 2 moles of hydrogen leftover.
From the given,
200 handle bars, 150 wheels, 250 pedals, and 75 seats.
For tricycle making, each one required one seat, handle,two pedals and three wheels.
Hence, wheels are first consumed.
Therefore, wheels are limiting reactant.