Answer:
22.98 is the most likely atomic weight of sodium
Step-by-step explanation:
Sodium is a mono-isotopic element and its atomic weight is determined solely by its isotope
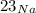 .Usually Atomic masses are not integers since they are taken as the average weight of the atomic mass of the isotope of the element that are occuring naturally. atomic weight of the atom depends on the 'atomic mass unit' (amu), that is 1/12th of the mass of carbon-12 present in its ground state. Electrons also contribute to the atomic mass.
.Usually Atomic masses are not integers since they are taken as the average weight of the atomic mass of the isotope of the element that are occuring naturally. atomic weight of the atom depends on the 'atomic mass unit' (amu), that is 1/12th of the mass of carbon-12 present in its ground state. Electrons also contribute to the atomic mass.