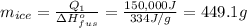Answer:
449.1 g
Step-by-step explanation:
The second law of thermodynamics states that heat flows from hotter objects to colder ones. Assuming that
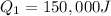 is the heat given off by a substance in order to melt the ice, we also need to introduce the equation representing the melting of ice:
is the heat given off by a substance in order to melt the ice, we also need to introduce the equation representing the melting of ice:
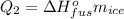
Since energy is conserved, the heat given off should be equal to the heat gained:
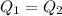
So that the equation becomes:
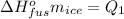
The enthalpy of fusion of ice is equal to:
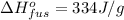
From here, rearrange the equation for the mass of ice: