Answer:
Tm = 1,728.38 °C
Step-by-step explanation:
mass of metal forging (Mm) = 67.2 kg
specific heat capacity of metal forging (Cm) = 438 J/kg°C
initial temperature of metal forging (Tm) = ?
final equilibrium temperature (Te) = 58.3 °C
mass of oil (Mo) = 786 kg
specific heat capacity of oil (Co) = 2950 J/kg°C
temperature of oil (To) = 37.1 °C
Mm × Cf × (Tm - Te) = Mo × Co × (Te - To)
Tm =
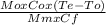 + Te
+ Te
Tm =
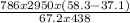 + 58.3
+ 58.3
Tm = 1,728.38 °C