Answer:
77362.56 J
163730.28571 J
Step-by-step explanation:
A = Area = 25 mm²
l = Length = 300 mm
K = Constant =
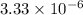
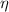 = Heat transfer factor = 0.75
= Heat transfer factor = 0.75
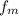 = Melting factor = 0.63
= Melting factor = 0.63
T = Melting point of low carbon steel = 1760 K
Volume of the fillet would be
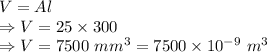
The unit energy for melting is given by
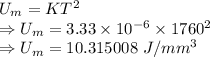
Heat would be

Heat required to weld is 77362.56 J
Amount of heat generation is given by
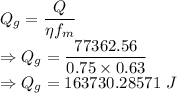
The heat generated at the welding source is 163730.28571 J