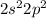Answer:
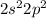
Step-by-step explanation:
Group 4A contains a total of 4 electrons for each atom in their valence shell. Filling the orbital diagram, let's say, for carbon, notice that when we start with period 2, we have two elements in the s-block, that is, lithium and beryllium. They correspond to the two s electrons that belong to the valence shell of carbon.
Moving on, we have boron and carbon, the remaining 2 electrons. Now, starting with boron, we're in the p-block.
That said, looking at the second period, the electron configuration for the valence shell of a group 4A element would be: