Answer:
0.4595 is the value of the equilibrium constant for the reaction.
Step-by-step explanation:
A ⇆ B
The forward direction, is first order in A with a rate constant = K =
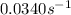
The rate of forward reaction = R
![R=K[A]](https://img.qammunity.org/2020/formulas/chemistry/high-school/difcblkjzgipdp2yew4xpfig6johj9d3qo.png)
The backward direction, is first order in A with a rate constant = K' =
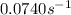
The rate of backward reaction = R'
![R'=K'[B]](https://img.qammunity.org/2020/formulas/chemistry/high-school/2s5sw38gcq9ouwa0uzi01izklke0mmr4tx.png)
At equilibrium rate of forward reaction are equal to the rate of backward reaction.
R = R'
![K[A]=K'[B]](https://img.qammunity.org/2020/formulas/chemistry/high-school/bqxyc4wlr1aukcqid7gu58vczhy5l04hxf.png)
![K_(eq)=([B])/([A])=(K)/(K')=(0.0340 s^(-1))/(0.0740 s^(-1))=0.4595](https://img.qammunity.org/2020/formulas/chemistry/high-school/fmc5lt4x15jinlgu3z9t2nbxgtmusxrwjz.png)
0.4595 is the value of the equilibrium constant for the reaction.