Answer : This reaction occur spontaneously at temperature above in kelvins is, 340 K
Explanation : Given,
 = 17 KJ/mole = 17000 J/mole
= 17 KJ/mole = 17000 J/mole
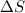 = 50 J/mole.K
= 50 J/mole.K
Gibbs–Helmholtz equation is :
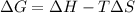
As per question the reaction is spontaneous that means the value of
 is negative or we can say that the value of
is negative or we can say that the value of
 is less than zero.
is less than zero.
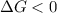
The above expression will be:
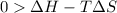
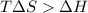
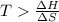
Now put all the given values in this expression, we get :
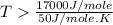
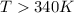
Therefore, this reaction occur spontaneously at temperature above in kelvins is, 340 K