Answer:
The equilibrium coefficient of the reaction is 0.0891.
Step-by-step explanation:
The balanced chemical reaction from the given data is as follows.
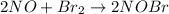
![K_(eq)=([NOBr]^(2))/([NO]^(2)[Br_(2)])](https://img.qammunity.org/2020/formulas/chemistry/middle-school/nwiov09tgxkpvn8oojfubq69alj9shhhz5.png)
From the given data,
[NOBr] = 0.0474 mol/L
[NO] = 0.312 mol/L
![[Br_(2)]=0.259 mol/L](https://img.qammunity.org/2020/formulas/chemistry/middle-school/4b81wtgulj47ipaf4kgv1uensvcl1jy8le.png)
Substitute the all given values into the
 formula.
formula.
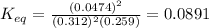
Therefore, The equilibrium coefficient of the reaction is 0.0891.