Answer:
Decomposition of aluminium oxide forms aluminium atoms and oxygen atoms.
Step-by-step explanation:
Decomposition reaction:
When a single compound break down into two or more simpler products.
For example "AB" reactant undergoes decomposition to form "A" and "B" products.
The chemical reaction is as follows.
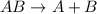
The given compound is aluminium oxide.
The decomposition reaction of aluminium oxide is a follows.
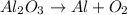
The balanced equation is as follows.
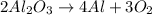
Therefore, Decomposition of aluminium oxide forms aluminium atoms and oxygen atoms.