Answer:
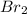
Step-by-step explanation:
The compound
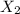 is
is
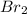 .
.
The reaction included are:
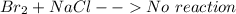 (because bromine is less reactive than chlorine)
(because bromine is less reactive than chlorine)
But , because of hexane the solution get dilute and its color changes to orange.
Now, NaI is added and we know Br is more reactive than I.
Therefore it replace it.
Reaction:
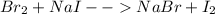
Purple color develop due to formation of Iodine.