Answer:
Radius of
 ion is more than
ion is more than
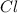
Step-by-step explanation:
Radius of atom : It depicts the size of the atom . Distance from the centre of the nucleus to the outermost shell of the atom.
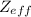 = Effective nuclear charge : It is the total positive charge of nucleus felt by the valence electron. Greater the
= Effective nuclear charge : It is the total positive charge of nucleus felt by the valence electron. Greater the
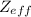 , smaller is the size of the atom ,since electron get held tightly toward nucleus.
, smaller is the size of the atom ,since electron get held tightly toward nucleus.
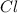 : It's radius is 99 pm (picometer) .It has atomic number 17 . 17 proton are holding 17 electrons
: It's radius is 99 pm (picometer) .It has atomic number 17 . 17 proton are holding 17 electrons
 : It's radius is 181 pm. 17 proton are holding 18 electrons
: It's radius is 181 pm. 17 proton are holding 18 electrons
Reason for increase in size from
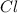 to
to
 .
.
 is formed when one electron is added to Cl atom.This causes more electron-electron repulsion within the shell.The attractive force of the nucleus reduce because
is formed when one electron is added to Cl atom.This causes more electron-electron repulsion within the shell.The attractive force of the nucleus reduce because
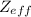 decreases.
decreases.