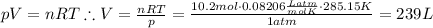Answer:
239 L
Step-by-step explanation:
1. Propane reacts with oxygen to produce carbon dioxide and water:
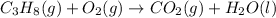
Firstly, 3 carbon atoms are required on the right:
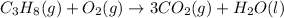
Secondly, 8 hydrogens in total (4 water molecules) are required on the right:
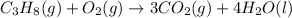
On the right, we have a total of 10 oxygen atoms, this implies we need 5 oxygen molecules on the left:
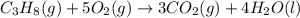
2. Calculate moles of propane using the the ratio of mass to molar mass:
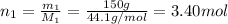
According to the stoichiometry, we have 3 times greater amount of carbon dioxide:
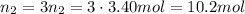
Use the ideal gas law to solve for volume: