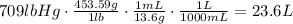Answer:
23.6 L
Step-by-step explanation:
Several conversion factors are needed to solve this problem. The following relationships are required:

Now, the density of mercury is:
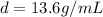
As well as another conversion factor between liters and milliliters:
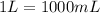
We might apply dimensional analysis problem solving technique for this problem. We start with lb and we wish to convert into grams first, this is done by multiplying lb by a factor of g/lb, so that pounds cancel out and we obtain grams.
Having grams, we'll multiply by a factor of mL/g to cancel out the grams and obtain milliliters.
After we have milliliters, we'll multiply by L/ml factor to convert into liters finally.
Putting all of this together, we obtain: