Answer:
q = 1,297 10⁻¹⁹ C , n=1
Step-by-step explanation:
For this problem we will use Newton's second law in the case of equilibrium
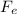 + B - W = 0
+ B - W = 0
Where
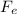 is the electrical force up, B the thrust and W the weight of the drop.
is the electrical force up, B the thrust and W the weight of the drop.
Let's look for weight and thrust
oil
ρ = m / V
m = ρ V
Air
B =
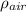 g V
g V
Electric force
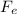 = qE
= qE
E = V / d
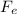 = q V/d
= q V/d
Let's replace
q V / d +
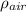 g V - ρ V g = 0
g V - ρ V g = 0
qV / d = (4/3 π r³) g (ρ –
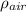 )
)
q = 4/3 π r³ (ρ –
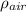 ) d / V
) d / V
Reduce to SI units
d = 1.87 cm (1m / 100cm) = 1.87 10⁻² m
ρ= 0.816 r / cm3 (1kg / 1000g) (102cm / 1m)³ = 816 kg / m³
 = 1.28 kg / m³
= 1.28 kg / m³
Let's calculate the charge
r = d / 2 = 3.3 10⁻⁶ m
r = 1.65 10⁻⁶ m
q = 4/3 π (1.65 10⁻⁶)³ (816 - 1.28) 0.0187 / 2210
q = 12.9717 10⁻²⁰ C
q = 1,297 10⁻¹⁹ C
If we assume that the load is
q = n e
In this case n = 1