Step-by-step explanation:
The given data is as follows.
m = 0.5 kg,
 ,
,

It is known that specific heat of aluminium is 0.91 kJ/kg.
As we know that, dQ = dU + dw
where, dQ = heat transfer
dU = change in internal energy
dw = work transfer
For the given system, work transfer "w" is 0.
(a) Hence, change in stored energy will be calculated as follows.
Q =
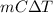
=

= 31.85 kJ
(b) The amount of heat transferred will be equal to change in stored energy.
So, dQ = Q = 31.85 kJ
(c) Change in entropy will be calculated as follows.
dS =

=

= 0.684 kJ/K
(d) Entropy transfer by heat will be calculated as follows.

=

= 0.1087 kJ/K
(e) Entropy change will be calculated as follows.
Entropy change = entropy transfer + entropy generation

0.684 kJ/K = 0.187 +

 = 0.5752 kJ/K
= 0.5752 kJ/K