Answer:
Step-by-step explanation:
Equation for hydrogen ion spectrum is as follows .
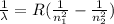
If n₁ = 1 and n₂ = 2,3,4 ...., it is called Layman series of radiation.
For first member n₁ = 1 and n₂ = 2,
R = 1.09735 x 10⁷ m⁻¹
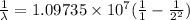
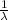 = .82301 x 10⁷
= .82301 x 10⁷
λ = 1.21505 x 10⁻⁷
= 1215.05x 10⁻¹⁰ m
1215.05 A
b ) For second member of Layman series
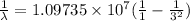
λ = 1.0252 x 10⁻⁷
= 1025.2x 10⁻¹⁰ m
1025.2A
For third member of Layman series
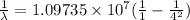
λ = .97204 x 10⁻⁷
= 972 x 10⁻¹⁰ m
= 972A
If n₂ = ∝ ( infinity)
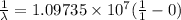
λ = .9113 x 10⁻⁷
= 911.3 x 10⁻¹⁰ m
= 911.3 A