Answer:
at n= 3 λ = 656 nm
at n= 2 λ = 121.58 nm
Step-by-step explanation:
Given details
transition of hydrogen atom from n = 2 to n = 3 state
Difference in energy between n = 3 state and n = 2 state :
![= 2.18*10^(-18) * [1/4 - 1/9] J = 3.03 * 10^(-19) J](https://img.qammunity.org/2020/formulas/physics/college/j3g6nzhnyklz0gp0j7qrrewqg7tt84c3jr.png)
so, energy of photon is given as
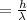
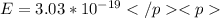 So solve for wavelength
So solve for wavelength
so, λ
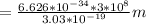
=
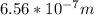
= 656 nm
for second transition,
energy transmitted is given asΔE
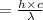
and it is calculated as
![= 2.18*10^(-18)*[1/1 -1/4] J](https://img.qammunity.org/2020/formulas/physics/college/hw4pqv14um27n6mkvdd1sm8l9z0eadttz6.png)
E = 1.635*10-18 J solving for wavelength in ENERGY equation we get
so,
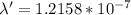 m
m
= 121.58 nm