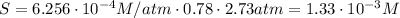Answer:
Step-by-step explanation:
(a) According to the Henry's law, the solubility is equal to the product between the Henry's law constant and the partial pressure of a gas:
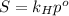
Air is at standard pressure:
![p = 1.00 atm[/atm]</p><p>Nitrogen's pecentage is:</p><p>[tex]\omega = 0.780](https://img.qammunity.org/2020/formulas/chemistry/college/49aygrsp8kaz4e4qm13tkaqpy04pa6l6bv.png)
Therefore, its partial pressure is:
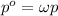
Solving for the Henry's constant:
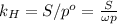
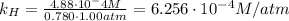
(b) Using the constant we've found in the previous part, we know that:
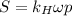
In this case, the percentage is kept the same, however, the total air pressure is:
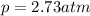
Substituting the variables: