Answer:
t = 17199 years
Step-by-step explanation:
given,
mass of sample = 1.09 Kg
Activity of living material = 15 decays / min /g
Activity of living material = 15 x 1000 decays /min /kg
Activity of living material per 1.09 kg A = 1.09 x 15 x 1000 decays / min
Activity of after time t is A ' = 2020
half life = 57300 years
desegregation constant
λ = 0.693 / 5700
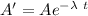
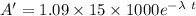
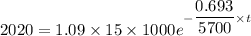
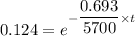
taking ln both side
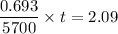
t = 17199 years