Answer:
4066 decay/s
Step-by-step explanation:
Given that:-
The weight of the person is:- 190 lb
Also, 1 lb = 453.592 g
So, weight of the person = 86182.6 g
Also, given that carbon is 18% in the human body. So,
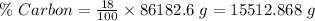
Carbon-14 is
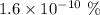 of the carbon in the body. So,
of the carbon in the body. So,
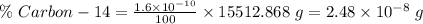
Also,
14 g of Carbon-14 contains
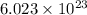 atoms of carbon-14
atoms of carbon-14
So,
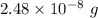 of Carbon-14 contains
of Carbon-14 contains
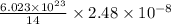 atoms of carbon-14
atoms of carbon-14
Atoms of carbon-14 =

Given that:
Half life = 5730 years
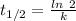
Where, k is rate constant
So,
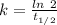
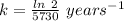
The rate constant, k = 0.00012 years⁻¹
Also, 1 year =
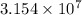 s
s
So, The rate constant, k =
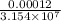 s⁻¹ =
s⁻¹ =
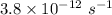
Thus, decay events per second =
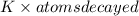 =
=
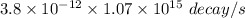 = 4066 decay/s
= 4066 decay/s