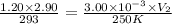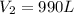Answer: The final volume of the balloon is 990 L
Step-by-step explanation:
Combined gas law is the combination of Boyle's law, Charles's law and Gay-Lussac's law.
The combined gas equation is,
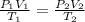
where,
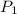 = initial pressure of gas = 1.20 atm
= initial pressure of gas = 1.20 atm
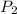 = final pressure of gas =
= final pressure of gas =
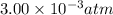
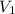 = initial volume of gas = 2.90 L
= initial volume of gas = 2.90 L
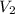 = final volume of gas = ?
= final volume of gas = ?
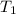 = initial temperature of gas =
= initial temperature of gas =
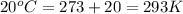
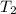 = final temperature of gas =
= final temperature of gas =
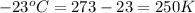
Now put all the given values in the above equation, we get: