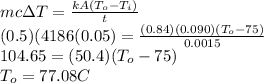Answer:
77.08 C
Step-by-step explanation:
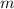 = mass of the water = 500 g = 0.5 kg
= mass of the water = 500 g = 0.5 kg
 = specific heat of water = 4186 J/(kg °C)
= specific heat of water = 4186 J/(kg °C)
 = Rate of change of temperature = 3 °C /min = (3/60 ) °C /s = 0.05 °C /s
= Rate of change of temperature = 3 °C /min = (3/60 ) °C /s = 0.05 °C /s
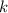 = thermal conductivity of glass = 0.84
= thermal conductivity of glass = 0.84
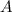 = Area of the element = 0.090 m²
= Area of the element = 0.090 m²
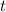 = thickness of the element = 1.5 mm = 0.0015 m
= thickness of the element = 1.5 mm = 0.0015 m
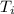 = Temperature inside = 75 °C
= Temperature inside = 75 °C
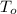 = Temperature outside = ?
= Temperature outside = ?
Using conservation of energy
Heat gained by water = Heat transferred through glass