Answer:
3396.53 years
Step-by-step explanation:
Using decay formula
In(
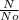 ) = -Kt where t is the age of the artifact in years and k is the decay constant
) = -Kt where t is the age of the artifact in years and k is the decay constant
T1/2 =
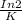
5730 =
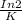
K = In 2 / 5730= 0.000121yr^-1
N / No = 0.663
In (0.663) / -0.000121 = t
t = 3396.53 years