The question is incomplete, here is the complete question:
Phosgene,
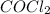 , gained notoriety as a chemical weapon in World War I. Phosgene is produced by the reaction of carbon monoxide with chlorine:
, gained notoriety as a chemical weapon in World War I. Phosgene is produced by the reaction of carbon monoxide with chlorine:
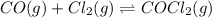
The value of
 for this reaction is 5.79 at 570 K. What are the equilibrium partial pressures of the three gases if a reaction vessel initially contains a mixture of the reactants in which
for this reaction is 5.79 at 570 K. What are the equilibrium partial pressures of the three gases if a reaction vessel initially contains a mixture of the reactants in which
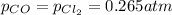 and
and
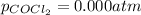 ?
?
Answer: The equilibrium partial pressure of CO,
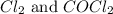 is 0.257 atm, 0.257 atm and 0.008 atm respectively.
is 0.257 atm, 0.257 atm and 0.008 atm respectively.
Step-by-step explanation:
The relation of
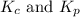 is given by:
is given by:
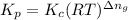
 = Equilibrium constant in terms of partial pressure
= Equilibrium constant in terms of partial pressure
 = Equilibrium constant in terms of concentration = 5.79
= Equilibrium constant in terms of concentration = 5.79
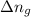 = Difference between gaseous moles on product side and reactant side =
= Difference between gaseous moles on product side and reactant side =
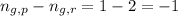
R = Gas constant =
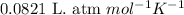
T = Temperature = 570 K
Putting values in above equation, we get:
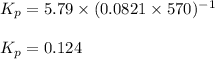
We are given:
Initial partial pressure of CO = 0.265 atm
Initial partial pressure of chlorine gas = 0.265 atm
Initial partial pressure of phosgene = 0.00 atm
The given chemical equation follows:
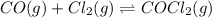
Initial: 0.265 0.265
At eqllm: 0.265-x 0.265-x x
The expression of
 for above equation follows:
for above equation follows:
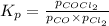
Putting values in above equation, we get:
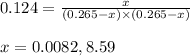
Neglecting the value of x = 8.59 because equilibrium partial pressure cannot be greater than initial pressure
So, the equilibrium partial pressure of CO =
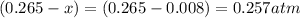
The equilibrium partial pressure of
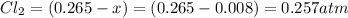
The equilibrium partial pressure of
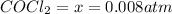
Hence, the equilibrium partial pressure of CO,
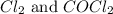 is 0.257 atm, 0.257 atm and 0.008 atm respectively.
is 0.257 atm, 0.257 atm and 0.008 atm respectively.