Answer:
0.244 M
Step-by-step explanation:
Calculation of the moles of glucose as:-
Mass = 11.0 g
Molar mass of glucose = 180.156 g/mol
The formula for the calculation of moles is shown below:

Thus,
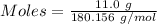
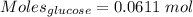
Molarity is defined as the number of moles present in one liter of the solution. It is basically the ratio of the moles of the solute to the liters of the solution.
The expression for the molarity, according to its definition is shown below as:

Given that:- Volume added = 100 mL = 0.1 L
So, Molarity of the glucose solution,
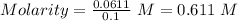
Considering
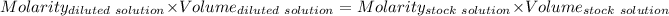
Given that:
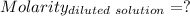
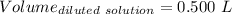
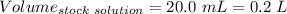
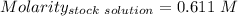
So,
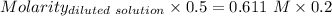
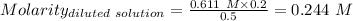
The molarity of the diluted solution is:- 0.244 M