Answer:
The number of neutron in the Aluminium Isotope is :
B. 14
Step-by-step explanation:
Isotopes : These are the atoms which have same atomic number but have different mass number.
This image shows the average atomic mass of Al element because it is in decimals.
Atomic mass = 26.98154
(Note : mass number of single isotope can never be in decimals)
It is the average of mass of different isotopes of Al
Major Isotopes of
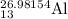 are :
are :
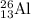 ......atomic mass = 26
......atomic mass = 26
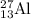 .......atomic mass = 27
.......atomic mass = 27
mass of Al given in image(26.98) is nearly equal to mass of 2nd isotope(27)
mass of
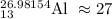
Now calculate the neutron in
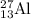
Number of neutron = mass number - atomic number
= 27 - 13
Number of neutron = 14
(Atomic mass is same as mass number)