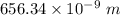Answer:
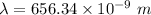
Step-by-step explanation:
Using the Rydberg formula as:
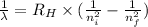
where,
λ is wavelength of photon
R = Rydberg's constant (1.097 × 10⁷ m⁻¹)
n₁ is the initial final level and n₂ is the final energy level
Given that:-
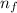 = 3
= 3
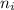 = 2
= 2
Applying in the formula as:
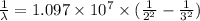
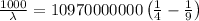
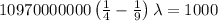
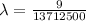
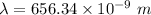
The wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=2 to an orbital with n=3 is:-