Answer:
(a) 0.26 % (b) 0.80 %
Step-by-step explanation:
(a)
Given that:

Concentration = 0.10 M
Considering the ICE table for the dissociation of acid as:-
![\begin{matrix}&HA&\rightleftharpoons &A^-&&H^+\\ At\ time, t = 0 &0.10&&0&&0\\At\ time, t=t_(eq)&-x&&+x&&+x\\ ----------------&-----&-&-----&-&-----\\Concentration\ at\ equilibrium:-&0.10-x&&x&&x\end{matrix}]()
The expression for dissociation constant of acid is:
![K_(a)=\frac {\left [ H^(+) \right ]\left [ {A}^- \right ]}{[HA]}](https://img.qammunity.org/2020/formulas/chemistry/college/i4f21r4h62kuq50o6c7vl3632ipxkz4f6s.png)
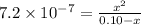
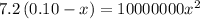
Solving for x, we get:
x = 0.00026 M
Percentage ionization =
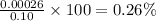
(b)
Concentration = 0.010 M
Considering the ICE table for the dissociation of acid as:-
![\begin{matrix}&HA&\rightleftharpoons &A^-&&H^+\\ At\ time, t = 0 &0.010&&0&&0\\At\ time, t=t_(eq)&-x&&+x&&+x\\ ----------------&-----&-&-----&-&-----\\Concentration\ at\ equilibrium:-&0.010-x&&x&&x\end{matrix}]()
The expression for dissociation constant of acid is:
![K_(a)=\frac {\left [ H^(+) \right ]\left [ {A}^- \right ]}{[HA]}](https://img.qammunity.org/2020/formulas/chemistry/college/i4f21r4h62kuq50o6c7vl3632ipxkz4f6s.png)
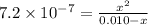
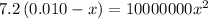
Solving for x, we get:
x = 0.00008 M
Percentage ionization =
