Answer:
C. all above is true.
Step-by-step explanation:
Energy releasing reactions are exothermic. Total energy of products (
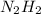 ) is less than the total energy of reactants (
) is less than the total energy of reactants (
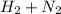 ) gives negative enthalpy change.
) gives negative enthalpy change.
hint: prefix exo- means "outside, external".