Answer:
+2
Step-by-step explanation:
If a compound
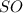 existed, we would identify the oxidation state of sulfur using the following logic:
existed, we would identify the oxidation state of sulfur using the following logic:
- oxygen is more electronegative than sulfur, so it's more electron-withdrawing and it should have a negative oxidation state producing a positive oxidation state for sulfur;
- oxygen typically has an oxidation state of -2;
- we may then apply the fact that SO is expected to be a molecule with a net charge of 0;
- if the net charge is 0 and the oxidation state of oxygen is -2, we may set the oxidation state of S to x;
- write the equation for the net charge of 0 by adding all individual charges of the two atoms:
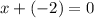 ;
; - hence, x = 2.
That said, in this hypothetical compound S would have an oxidation state of +2.