Answer:
She needs 1.19 moles of potassium hydroxide.
Step-by-step explanation:
Being the molar mass of the elements:
- K= 39 g/mole
- O= 16 g/mole
- H= 1 g/mole
then the molar mass of potassium hydroxide is:
KOH= 39 g/mole + 16 g/mole + 1 g/mole= 56 g/mole
Being the mass of one mole of a substance, which can be an element or a compound, you can apply the following rule of three: if 56 g of KOH are present in 1 mole, 66.48 g of KOH in how many moles of the compound are they?
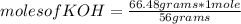
moles of KOH= 1.19
She needs 1.19 moles of potassium hydroxide.