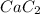Answer:
Step-by-step explanation:
Given that:-
1 calcium ion is at each corner. Also, 1 corner of the unit cell is shared by 8 unit cubes. So, the share of a tom in one unit cell is:-
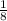
Also, there are 8 corners in one unit cell.
So, Total number of atoms at corners =
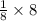 = 1
= 1
Number of calcium atoms in the unit cell = 1
Also, given that there are two carbons which are present internally in the unit cell, So,
Number of carbon atoms in the unit cell = 2
Hence, the formula of the calcium carbide is:-