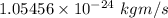Answer:
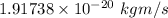
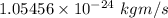
Step-by-step explanation:
h = Planck's constant =
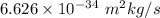
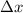 = Uncertainty in the position
= Uncertainty in the position
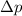 = Uncertainty in the momentum
= Uncertainty in the momentum
From the uncertainty principle
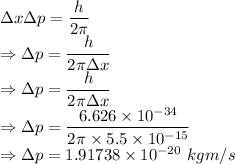
The minimum uncertainty in the momentum of the proton is
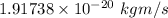
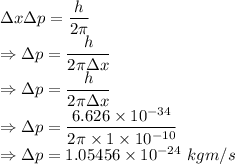
The minimum uncertainty in the momentum of the electron is