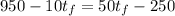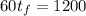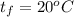Answer:
Between
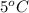 and
and
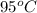
Step-by-step explanation:
According to the second law of thermodynamics, heat flows from hotter objects toward colder objects.
Having a mixture of two components, one at a higher temperature of
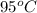 and the other one at a lower temperature of
and the other one at a lower temperature of
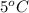 , heat will flow from the first object to the second one.
, heat will flow from the first object to the second one.
The temperature of the first object will decrease and the temperature of the second object will increase. Assuming no phase change, the final equilibrium temperature will be reached between
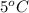 and
and
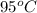 .
.
We cannot tell the exact temperature, unless we know the specific heat capacity of each material and their masses. The first object will give off heat given by:
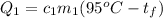
The second object will gain this heat by:
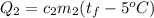
Since the heat given off is equal to the heat gained:
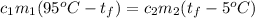
Let's say that each object is water with a specific heat capacity of:
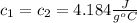
The mass of the first object is 10 g, the mass of the second one is 50 g, then:
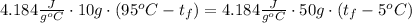
The equation is simplified to: