Answer:
Decrease, decrease, 323 °C
Step-by-step explanation:
Suppose a sample of an ideal gas in a container is subjected to a temperature change. A decrease in temperature will decrease the kinetic energy and average speed of the gas particles. As a result, the pressure on the walls of the container will decrease. If the gas starts at 25 °C, what temperature would the gas need to reach for its pressure to double?
The initial temperature is 25°C + 273 = 298 K. We want the pressure P₂ to be the double of the pressure P₁. We can find the required temperature T₂ using Gay-Lussac's law.
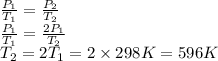
T₂ = 596 K - 273 = 323 °C