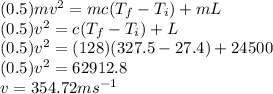Answer:
354.72 m/s
Step-by-step explanation:
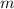 = mass of lead bullet
= mass of lead bullet
 = specific heat of lead = 128 J/(kg °C)
= specific heat of lead = 128 J/(kg °C)
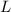 = Latent heat of fusion of lead = 24500 J/kg
= Latent heat of fusion of lead = 24500 J/kg
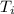 = initial temperature = 27.4 °C
= initial temperature = 27.4 °C
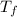 = final temperature = melting point of lead = 327.5 °C
= final temperature = melting point of lead = 327.5 °C
 = Speed of lead bullet
= Speed of lead bullet
Using conservation of energy
Kinetic energy of bullet = Heat required for change of temperature + Heat of melting